Launched new coronavirus SARS-CoV-2 inactivated solution 2
-Inactivates the virus 5 minutes after mixing saliva samples. It can be stored at room temperature. ~
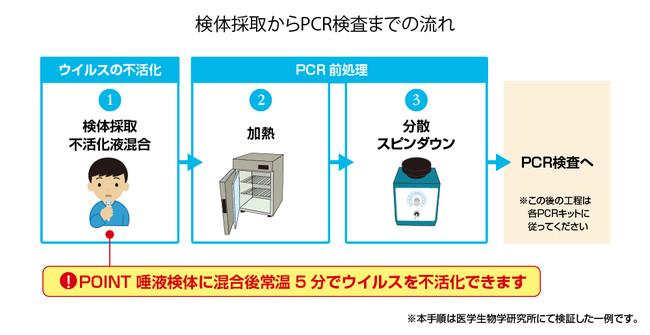
Click here for detailed product information of Medical & Biological Laboratories Co., Ltd. → https://lp.mblbio.com/ja/virus_inactivation_solution.html?pr20220117 Click here for orders and inquiries → https://ivd.mbl.co. jp / diagnostics / inq / index.html? pr20220117 Medical & Biological Laboratories, Inc. (President: Kimimasa Yamada, hereinafter MBL) will release MEBRIGHT ™ virus inactivated solution 2 today from January 17, 2022. Did. This new coronavirus inactivating reagent is a reagent developed to reduce the risk of infection for sample collectors, transporters, and measurement workers for PCR tests. Product features: Product information: [Reagents for research] Product name / Product code: MEBRIGHT ™ virus inactivating solution 2 (Code: GS-J0232) Desired delivery price: 14,000 yen (tax excluded) Packaging: 2.0 mL x 50 bottles Storage method: 2 to 25 ° C * This product is a research reagent. Do not use for diagnostic or other medical purposes. How to use: The operation of MEBRIGHT ™ virus inactivating solution 2 is only virus inactivating treatment (5 minutes after mixing) and RNA homogenization before measurement (vortex). * Pretreatment may be required depending on the PCR kit used. Main equipment / equipment: Equipment / equipment required for operating the virus inactivating solution * The equipment / equipment required differs depending on the pretreatment protocol, so prepare appropriate equipment. Example when using Ampdirect ™ 2019-nCoV detection kit (Shimadzu) ① Heat treatment / constant temperature device: Incubator, heat block, water bath, etc. that can heat at 70 ° C ② Uniformization / vortex mixer ③ Spindown / Centrifuge: A tube that can set saliva sample tubes, centrifuge tubes, etc. * No special specifications such as temperature control or high-speed rotation are required. Development background: While PCR tests for the new coronavirus have become widespread, the risk of infection to sample collectors, transporters, and PCR workers has become a problem. Especially when transporting infectious specimens, "inactivation treatment" is required to invalidate the infectivity of the virus in the specimens. Therefore, we participated in the framework of the AMED virus and other infectious disease control technology development business, worked on the development of a virus inactivated solution that can be used by the direct PCR method, and succeeded in the development of the MEBRIGHT ™ virus inactivated solution in July 2021. I did. We are pleased to announce the release of MEBRIGHT ™ Virus Inactivated Liquid 2, which has been improved to improve performance and usability, on January 17, 2022. Performance data (1): Inactivation test for SARS-CoV-2 The virus inactivation ability of this product was evaluated for SARS-CoV-2 prepared from cultured cells. A virus solution having a virus titer of 4.5x10⁷ (/ mL) was mixed with twice the amount of this product or physiological saline (control product) and allowed to stand at 25 ° C for 5 minutes. After that, the detergent was removed with a column, and 40 μL of each diluted solution, which was serially diluted 10-fold to 10⁸-fold, was inoculated into 6 wells inoculated with equal volumes of Vero E6 cells. After culturing for 4 days, the virus titer TCID₅₀ (/ mL) was calculated by counting the wells in which cytopathic effect due to virus infection was observed. As a result, as shown in the figure below, the virus titer was maintained when physiological saline was used, but the virus titer was below the detection limit when this product was used. Therefore, it was confirmed that SARS-CoV-2 is inactivated under the above conditions. [Fig. 1] Verification of inactivating infectivity * This test was conducted by Professor Shigeru Morikawa, Department of Microbiology, Faculty of Veterinary Medicine, Okayama University of Science, based on a joint research agreement with Kakei Gakuen. Performance data (2): Verification of RNA stability We evaluated the stability of RNA in a mixture of saliva sample and this product. A saliva sample containing inactivated SARS-CoV-2 added to 5.0 × 10² copy / μL was mixed with 2 times the amount of this product and allowed to stand at 4 ° C or 25 ° C for 0, 3 or 7 days. , Virus RNA was detected using Ampdirect ™ 2019-nCoV detection kit (Shimadzu Corporation). As shown in the figure below, we confirmed the stability of RNA in the mixed solution of saliva sample and this product when stored for up to 7 days at both 4 ° C and 25 ° C. [Fig. 2] Verification of RNA stability Please refer to the following for orders and inquiries. https://ivd.mbl.co.jp/diagnostics/inq/index.html?pr20220117 Please refer to here for the new corona special site of Medical & Biological Laboratories, Ltd. https://www.mbl.co.jp/novelcoronavirus/?pr20220117 Introducing PCR test and automatic antibody measurement device Established in 1969 as the first antibody manufacturer in Japan, it is now not only in the immunological field but also in genes. We are expanding our business to the field of diagnosis, researching, developing, manufacturing and selling clinical test reagents and basic research reagents. In the clinical test drug business, we develop and sell test drugs for autoimmune diseases, cancer, infectious diseases, etc. In the field of autoantibody diagnosis, we are expanding our product lineup as a top manufacturer in Japan and contributing to medical care in this field where there are many intractable diseases. In the field of cancer diagnosis, we are contributing to personalized medicine by developing companion diagnostics that predict the effects of drugs.Medical & Biological Laboratories Co., Ltd. Headquarters location 〒105-0012 2-11-8 Shibadaimon, Minato-ku, Tokyo Sumitomo Real Estate Shibadaimon 2-chome Building Phone 03-6854-3613 Email kensa@mbl.co.jp Academic Department and above- Press Release>
- Medical & Biological Laboratories Co., Ltd.>
- Launched new coronavirus SARS-CoV-2 inactivated solution 2